| Next month (March), when thousands of mixed martial arts fans converge on a ONE Championship event, they will have to do pre-event testing for Covid-19. Apart from healthcare personnel on site at the Singapore Indoor Stadium to oversee the testing, there will be 10 kiosks (or ATMs) to which many fans will be directed to do Antigen Rapid Tests (ARTs). Following on-screen instructions, they scan their IC or passport, do their nasal swaps, then insert the cassette into the ATM, and go off. The entire process is video-recorded and takes no more than 5 minutes.  The Straits Times, 15 Feb 2022Several minutes later, the test results are photographed inside the ATM and automatically sent by wi-fi to their phones and by e-mail to the event organiser. The Straits Times, 15 Feb 2022Several minutes later, the test results are photographed inside the ATM and automatically sent by wi-fi to their phones and by e-mail to the event organiser.In the meantime, after the previous user has stepped away from the ATM, another user can go through the same process for his test. The patent-pending ATMs, currently being trialled by two healthcare groups as reported recently by The Straits Times (above), were designed by Eddie Chng, CEO of Singapore-listed DISA Ltd. |
This graphic illustrates clear benefits of the ATM solution compared to how things are done now:
The ATM looks set to be the second of Eddie Chng's notable innovations, or what might be called disruptive innovations.
The first is a security solution known as Point-of-Sale Activation, which has been adopted by Walmart, the largest retailer in the world.
The solution is found in its 5,000 stores across the US for a range of electronic products, resulting in millions of dollars of savings a year from fraud. (Read more here)
Speaking with NextInsight, Eddie said the ATM-for-mass-testing idea germinated in his mind after he was struck by scenes of mass-testing using ART kits at workers' dormitories.
The process was cumbersome as it involved large numbers of staff, lots of administrative work to record results, and potentially could even be faked.
With his background in automation and engineering, he started to design the ATM and had 4 prototypes produced in China.
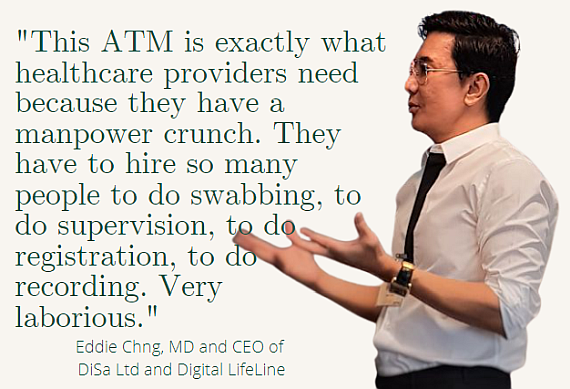
"This is exactly what healthcare providers need because they have a manpower crunch. They have to hire so many people to do the swabbing, to do supervision, to do registration, to do recording. Very laborious."
Fortuitiously, towards the end of 2021, he heard about a breakthrough by top Singapore institutions -- SingHealth Duke-NUS Academic Medical Centre and the National University of Singapore -- in developing saliva-based ART kits.
The tests are said to have high accuracy rates matching that of the gold standard, PCR tests.
Eddie reached out to the scientists (or principal investigators as they are also known) and won them over with his idea that the saliva-based kits be commercialised along with the ATMs. Don't go down the route of just selling the kits to Watson's and the like, he told them.
Thus, Digital Life Line Pte. Ltd. was set up as a subsidiary of DiSa to carry out the business after it signed an agreement with the co-developers for exclusive rights to manufacture and distribute the new kits.
 Milestone: Working fast, Digital Life Line approached a manufacturer in China to scale up what is a lab product into a commercial product. Milestone: Working fast, Digital Life Line approached a manufacturer in China to scale up what is a lab product into a commercial product.So, early next month (March), a maiden batch of 3,000 saliva test kits is scheduled to arrive in Singapore, says Eddie. The kits will be sent to the Ministry of Health for tests and approval. Some kits will also be sent to regional countries to seek regulatory approval for sale there as well. CE certification in order to sell in Europe will also be sought. |
Being large providers of ART test kits, Raffles Medical Group and Fullerton Health decided to trial the ATMs for a month.
Eddie sees such medical groups being key to the proliferation of the ATMs, since the medical groups are the ones appointed to oversee tests at border crossings, major events, ports, cruise centres, etc.
Such medical groups could also deploy the ATMs in their overseas markets.
In his business vision, Eddie sees medical groups purchasing the ATMs (at "below $10,000 a unit" -- a price equivalent to, say, the monthly salaries of two healthcare assistants): "This is to become a permanent tool for them to augment their services, just like they would buy a hospital bed or wheelchair instead of leasing them."
The medical groups will also be motivated to buy test kits (be it saliva-based or otherwise) from Digital Life Line.
He indicates that the pricing will be below what ART kits in the market sell for.
Digital Life Line's outsourced manufacturing capacity is potentially 700,000 kits a week.
| Aside from one-off ATM sales and recurring revenue from test kits, an integral part of the business model is to serialise the ART kits in order to accurately tag a kit to a specific user. The serialisation will be done by parent company DiSa in the unique way that it currently does for electronic goods supplied by OEMs to Walmart.  Prof Soo Khee Chee: Inaugural Benjamin Shears Professor in Academic Medicine at Duke-NUS Medical School.DiSa has a 40% stake in Digital Life Line. The bulk of the rest of the shareholding is held by Professor Soo Khee Chee (43%), Dr Melvin Chua (4%) and Dr Guy Heathers (1%). See details here. Prof Soo Khee Chee: Inaugural Benjamin Shears Professor in Academic Medicine at Duke-NUS Medical School.DiSa has a 40% stake in Digital Life Line. The bulk of the rest of the shareholding is held by Professor Soo Khee Chee (43%), Dr Melvin Chua (4%) and Dr Guy Heathers (1%). See details here. It will not be surprising if these stakes are diluted given that Digital Life Line is likely to tap the capital market to support the intial ramp-up of its business. With the pieces of the story falling into place, will Singapore see a sea change in the mass-testing process here -- and possibly overseas? |







